Fluid transfer plays a crucial role in bioprocessing, which involves the movement of various liquids, such as media, buffers, and harvested bioproducts, between different process steps. Maintaining sterility, preventing cross-contamination, and ensuring efficient and reliable fluid transfer is essential for successful bioprocess operations. Connecting the pieces from start to finish in an aseptic way can be a difficult task. This blog will discuss some of the best practices in fluid transfer for bioprocessing, focusing on techniques, equipment, and considerations to achieve optimal results.
1.Proper Equipment Selection:
Choosing the right equipment for fluid transfer is fundamental to ensure the integrity of the process. Consider the following factors:
a. Material Compatibility: Select materials that are chemically compatible with the fluid being transferred to prevent contamination or adverse reactions. Also, be aware of reactions between materials that could compromise performance. Understanding the material compatibility between silicone and TPE (thermoplastic elastomer) tubing is crucial. By conducting appropriate compatibility studies, you can make informed decisions regarding the selection of silicone or TPE tubing for their specific application requirements.
b. Sterilizability: Use equipment that holds up to your chosen sterilization modality or understand effects that may impact the performance of a material or component.
c. Ease of Use: Opt for user-friendly equipment, facilitating the efficient and error-free fluid transfer.
2.Establishing Aseptic Techniques:
Maintaining sterility throughout the fluid transfer is critical to avoid microbial contamination. Adhere to these guidelines:
a. Clean Working Environment: To minimize the risk of contamination, perform fluid transfer operations in a designated clean area, such as a laminar flow hood or an ISO Class 5 cleanroom.
b. Personal Protective Equipment (PPE): Wear appropriate PPE, including gloves, lab coats, masks, and hairnets, to prevent the introduction of contaminants from personnel.
c. Disinfection: Thoroughly clean and disinfect all equipment and surfaces involved in fluid transfer, including tubing, connectors, and transfer vessels, using appropriate disinfectants.
3.Proper Handling and Storage:
To maintain the quality and viability of fluids during transfer, consider the following practices:
a. Temperature Control: To preserve their integrity, keep temperature-sensitive fluids within their specified temperature range during transfer and storage.
b. Minimize Exposure: Minimize exposure to air, light, and other environmental factors that may degrade the fluid’s quality. Use opaque tubing or cover transfer vessels to shield light-sensitive fluids. In addition, keeping your tubing and hose organized and off the floor using a Hose Holder reduces the potential for accidental contamination, as the floor is the dirtiest part of the clean room.

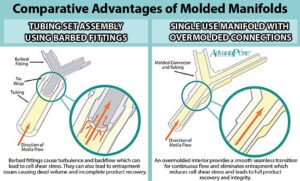
c. Minimize Shear Stress: Avoid excessive agitation or turbulence during fluid transfer to minimize shear stress, which can damage delicate cells or proteins. The best way to do that is to have smooth junctions using overmolding. Overmolding reduces the potential for shear stress and the likelihood of leaks and accidental contamination because the system is one unit versus multiple components with barbed junctions.
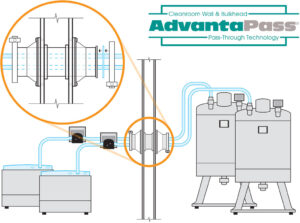
d. Moving From Room To Room: One place there is a lot of risk for contamination is when moving fluid from room to room. AdvantaPass provides a safe and easy way to move large volumes of liquid from one room to another without the risk of bin breakage or spillage.
4.Validation and Documentation:
Maintaining thorough documentation and validation of fluid transfer processes is crucial for regulatory compliance and process optimization:
a. Standard Operating Procedures (SOPs): Develop and follow SOPs for fluid transfer operations, including step-by-step instructions, safety guidelines, and quality checks.
b. Equipment Calibration: Regularly calibrate equipment, such as pumps and flow meters, to ensure accurate and precise fluid transfer.
c. Process Monitoring: Implement robust monitoring systems to track critical process parameters, such as flow rates, pressures, and temperatures, to ensure consistency and identify any deviations.
5.Training and Continuous Improvement:
Invest in training programs and foster a culture of continuous improvement to enhance fluid transfer practices:
a. Operator Training: Provide comprehensive training to personnel involved in fluid transfer, emphasizing aseptic techniques, equipment handling, and safety protocols.
b. Performance Analysis: Analyze process data, evaluate performance metrics, and conduct periodic audits to identify areas for improvement and implement corrective actions.
c. Knowledge Sharing: Encourage collaboration and knowledge sharing among team members to leverage collective expertise and stay updated with the latest advancements in fluid transfer technology.
In conclusion, implementing best practices in fluid transfer for bioprocessing is essential to ensure the process’s integrity, sterility, and efficiency. By selecting appropriate equipment, adhering to aseptic techniques, and focusing on proper handling, storage, validation, and training, bioprocessing facilities can minimize the risk of contamination, improve process outcomes, and maintain high-quality bioproducts. With continuous improvement and attention to detail, bioprocess operators can enhance their fluid transfer practices and contribute to the success of their bioprocessing operations.
If you wish to talk about solutions with someone from our team phone us at 800-506-3924 or +1-215-526-2300,
e-mail info@newageindustries.com,
or use the Contact Us form below.