Verigenics GammaTag
Automated Identification Solutions Using RFID Technology
Verigenics offers solutions to enable medical devices, pharmaceuticals, and other regulated companies to trace and authenticate consumables. Verigenics products use RFID (Radio Frequency Identification) technology to provide enhancements such as increased safety for providers and patients, brand loyalty, increased productivity, inventory visibility, and improved product security.
![]()
The next generation of RFID is here – GammaTag RFID tags from Verigenics. They’re the first radio frequency identification tags that handle gamma radiation with no loss of data.
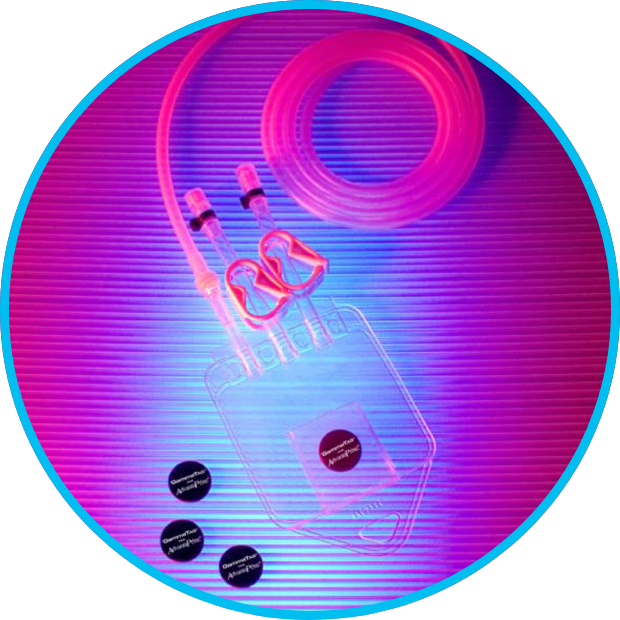
GammaTag provides reliable electronic data storage for single-use medical devices, bioprocess components, and other parts from inception to disposal. GammaTag is available exclusively from Verigenics, a division of NewAge® Industries.
Features
- Uses read/write RFID technology to identify critical process components in medical device, pharmaceutical, bioprocess/biomedical, food, and beverage industries.
- Record and access the current status of process components on the spot or use them simply for identification (part number, lot number, gamma sterilization date, etc.)
- All critical packaging and labeling documentation resides on the component throughout its useful life.
- Attaches to single-use medical devices, sample and production bags, tanks, filters, manifolds, tubing and hose, storage containers, single-use systems, boxes or pallets undergoing gamma radiation sterilization, and dosimeters
- Allows gamma radiation sterilization of a complete single-use system for the cleanest possible products
- It also withstands CIP sterilization processes.
- GammaTag’s read/write ability makes it unique – data may be written directly on the tag, unlike read-only barcode labels
- Provides reliable identification without the potential hazards of leachable found in label adhesives and permanent markers
- Will not fall off during cold storage like labels can
- Unlike bar code labels, GammaTag does not require a clear sight line for reading or writing
- Electronically links to notes, cleaning schedules, files, certifications, photos and illustrations, installation instructions, warning notices, disposal procedures, and other instructions
- Eliminates the burden and bulk of paper records and log books
- RoHS compliant
- U.S. Patent 8,519,846
- Field testing is recommended for each application
Applications
Single-Use Systems • Sample & Production Bags • Manifolds • Filters • Tubing & Hose • Storage Containers
• Boxes or Pallets Undergoing Gamma Radiation Sterilization • Dosimeters • Medical Devices
Specifications
| Dimensions: | 22mm in diameter; 2mm thick |
| Read/Write Range: | up to 50mm |
| Power Type: | passive; energized by RFID reader/writer |
| Gamma Radiation: | upto45kGy |
| Chipset: | Fujitsu MB89R118 |
| Memory Capacity: | 2048 bytes |
| User Memory Area: | 2000 bytes |
| Operating Frequency: | 13.56MHz ± 0.5MHz |
| Modulation Type: | 10%ASK |
| Operating Temperature: | -20°C to 85°C |
| Storage Temperature: | -40°C to 85°C |
| Data Retention Period: | 10 years at 55°C |
| Data Endurance: | 1010 cycles |
| Read Speed: | 1525 ms (2048 bytes) |
| Write Speed: | 1413 ms (2000 bytes) |
| Other: | 8 bytes/block configuration ISO/IEC 15693 commands full support Reading up to 258 blocks using custom commands |


